At Heron, we are thinking differently about care for oncology patients with innovative therapies that represent true advancement and make a meaningful difference in patient lives.
Our products aim to help manage and reduce the nausea and vomiting that happen right away or later with certain anticancer medicines (chemotherapy). Our goal is to deliver medicines that matter so that people with cancer and their families can spend more time doing the things they enjoy with the people that matter.
Nausea and Vomiting Are Common Side Effects of Chemotherapy
Chemotherapy-induced nausea and vomiting (CINV) is one of the most common and feared side effects experienced by patients with cancer.1 Despite the substantial progress in treatments to prevent CINV, as many as 80% of patients with cancer still experience nausea, vomiting, or both following receipt of chemotherapy.2
CINV is often attributed as a leading cause of premature discontinuation of cancer treatment.
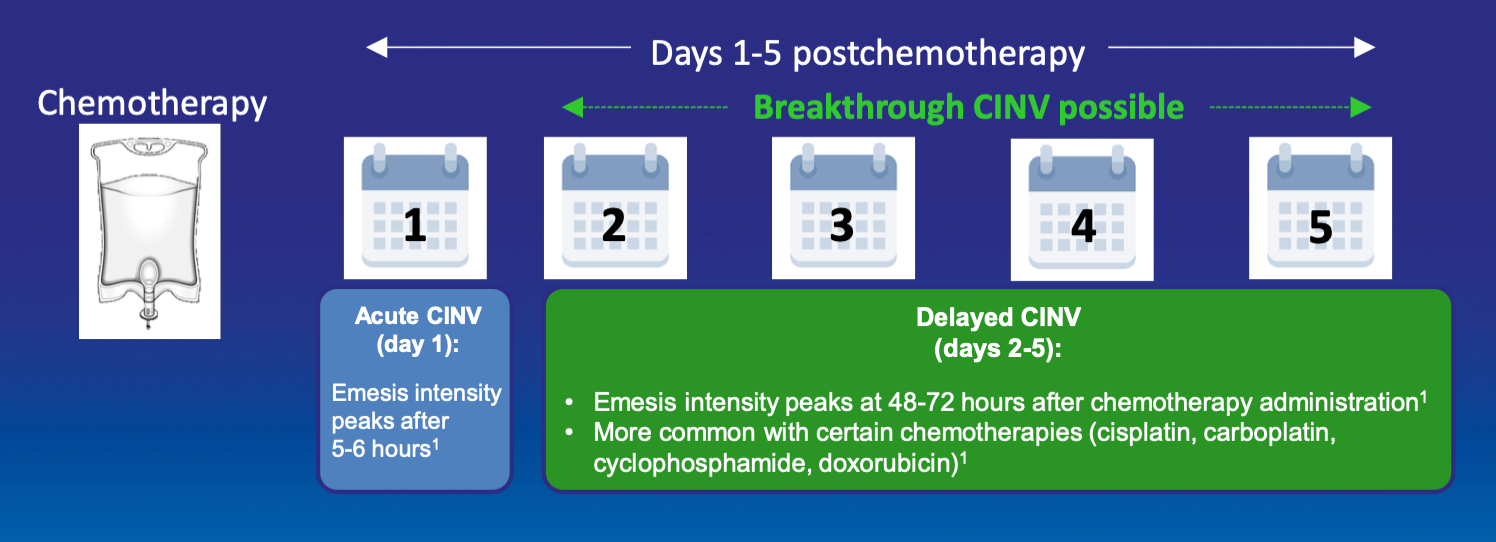
Types of CINV
- Acute CINV: occurs within day 1 after administration of chemotherapy
- Delayed CINV: occurs from days 2-5 after administration of chemotherapy
- Breakthrough CINV: occurs despite prophylactic treatment and may require the use of rescue medication
Most chemotherapy agents cause some degree of nausea and vomiting. However, the chemotherapy agents that cause the worst degree of nausea and vomiting are categorized into 2 groups: moderately emetogenic chemotherapy (MEC) and highly emetogenic chemotherapy (HEC).3 Thirty to 90% of patients undergoing MEC and more than 90% of patients undergoing HEC experience vomiting without preventative treatment.4,5
COMMON ANTICANCER AGENTS | By emetogenic category*†6
| EMETOGENIC POTENTIAL OF PARENTERAL ANTICANCER AGENTS | ||
| LEVEL | AGENT | |
| Highly emetogenic anticancer agents (>90% frequency of emesis) |
|
|
| Moderately emetogenic anticancer agents (>30%–90% frequency of emesis) |
|
|
| EMETOGENIC OF ORAL ANTICANCER AGENTS‡ | ||
| LEVEL | AGENT | |
| Moderately to highly emetogenic anticancer agents (≥30% frequency of emesis) |
|
|
*Low emetogenicity anticancer agent regimens not listed.
†These agents may be highly emetogenic in certain patients.
‡ For some moderately to highly emetogenic oral anticancer agents, routine antiemetic premedication may not be required.
aEmerging data and clinical practice suggest adding low-dose olanzapine and/or NK1 RA or 5-HT3 RA for nausea prevention
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis V.1.2021 © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available.
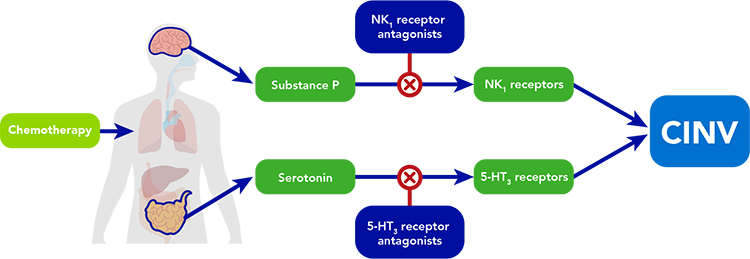
Two of the systems that regulate the body’s emetic response are the 5-hydroxytryptamine (5-HT3) receptor system and the neurokinin-1 (NK1) receptor system.
- Chemotherapy triggers the release of 5-hydroxytryptamine (5-HT) (serotonin), which acts by stimulating 5-HT3 receptors on neurons in the gastrointestinal tract and stimulating 5-HT3 receptors in the brain that control vomiting.
- Chemotherapy also causes the release of a molecule known as substance P, which acts on the NK1 receptors in the brain to reinforce the desire to vomit.
Along with other central and peripheral neurotransmitters, these systems work in concert to escalate the sensation of nausea and induce vomiting, constituting the body’s natural reflex to try to protect itself from foreign toxins. 5-HT3 receptor antagonists and NK1 receptor antagonists act synergistically on 2 of the critical pathways involved in the vomiting reflex to alleviate one of the key treatment-limiting side effects of chemotherapy.
The Impact of CINV May Not Be Fully Recognized
CINV is associated with serious consequences including:
- Dehydration, which can lead to increased resource utilization5,7
- Delays in treatment or discontinuation of future chemotherapy cycles8,9
- Risk for medical complications such as malnutrition and electrolyte imbalances10
- Increased utilization of healthcare resources such as visits to the physician’s office or hospital7,10
- Impact on quality of life by increasing the potential for hospitalizations and unscheduled visits for hydration5,7
Additionally, poorly managed CINV can put patients at risk for infection, requiring intervention and unscheduled office visits7,11,12 Learn more about our products for CINV.
View References
- Grunberg SM. Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment–how are we doing?. J Support Oncol. 2004;2(1 Suppl 1):1-11.
- Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Health Drug Benefits. 2012;5(4):232-240.
- Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4197.
- Kris M, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932-2947.
- Navari RM, et al. Unscheduled hydrations: redefining complete response in chemotherapy-induced nausea and vomiting studies. Future Oncol. 2020;16(24):1863-1872. PMID: 32543309.
- PDQ® Supportive and Palliative Care Editorial Board. PDQ nausea and vomiting related to cancer treatment. National Cancer Institute. Updated July 23, 2020. Accessed December 22, 2020. https://www.cancer.gov/about-cancer/treatment/side-effects/nausea/nausea-hp-pdq PMID: 26389491.
- Burke TA, et al. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131-140. doi:10.1007/s00520-009-0797-x
- Van Laar ES, et al. Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Support Care Cancer. 2015;23(1):151-157. doi:10.1007/s00520-014-2325-x
- Neymark N, et al. Impact of emesis on clinical and economic outcomes of cancer therapy with highly emetogenic chemotherapy regimens: a retrospective analysis of three clinical trials. Support Care Cancer. 2005;13:812-818. https://doi.org/10.1007/s00520-005-0803-x
- Schwartzberg L, et al. Resource utilization for chemotherapy-induced nausea and vomiting events in patients with solid tumors treated with antiemetic regimens. Am Health Drug Benefits. 2015;8(5):273-282.
- Soefje SA. Strategies to improve CINV outcomes in managed care. Am J Manag Care. 2018;24(18 suppl):S398-S404.
- Thom KA, et al. Infection prevention in the cancer center. Clin Infect Dis. 2013; 57(1):579-585. https://doi.org/10.1093/cid/cit290
Sign-Up for Email Updates
Receive news and updates on Heron’s latest innovations.